The leader
in prognostic melanoma testing
Guide your melanoma management with the most clinically validated and comprehensive GEP test on the market.
What is DecisionDx-Melanoma?
[.text-color-brand-blue]DecisionDx-Melanoma is the only melanoma gene expression profiling (GEP) test associated with improved survival.1[.text-color-brand-blue]
The test provides personalized results for patients with stage I-III cutaneous melanoma, guiding risk-aligned management decisions. By integrating the GEP score with traditional clinicopathologic features, DecisionDx-Melanoma results provide comprehensive results to answer two critical questions:
Clinically validated in the largest real-world study of GEP testing in melanoma
[.underline-move-left]Recent data[.underline-move-left] from an ongoing collaboration with the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program show that DecisionDx-Melanoma testing was associated with lower melanoma-specific and overall mortality compared to untested patients. The study confirms DecisionDx-Melanoma delivers significant, independent risk stratification beyond AJCC8 staging.1

Independently validated. Clinically tested.
If you're going to use a GEP test in clinical practice, it's critical to ensure that the test provides clear, independent value beyond what's already freely and readily available to you today—namely, American Joint Committee on Cancer (AJCC) staging.
DecisionDx-Melanoma has nine peer-reviewed studies demonstrating consistent, independent performance – no other GEP test in melanoma has such evidence.

Sentinel lymph node biopsy guidance you can trust
A recent study by Peter A. Prieto, MD, MPH, Laura K. Ferris, MD, PhD, and J. Michael Guenther, MD, published in Cancer Diagnosis & Prognosis, compared 31-GEP and CP-GEP for SLNB decision-making, analyzing five CP-GEP validation studies and four 31-GEP studies in T1-T2 tumors.
Overall, that data show that CP-GEP did not perform as well as AJCC, while DecisionDx-Melanoma performed better than AJCC4
Ultimately, “low risk” CP-GEP results are simply not low enough.
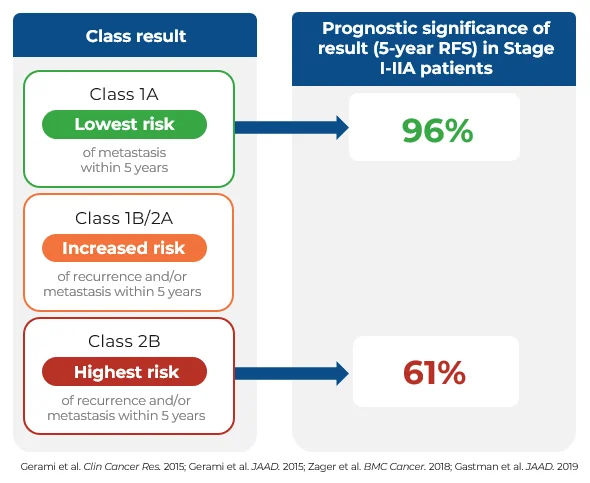
DecisionDx-Melanoma provides significant risk-stratification to inform management decisions
The visual on the right highlights the meaningful risk stratification between a Class 1A (lowest risk) and Class 2B (highest risk) DecisionDx-Melanoma result.
How does DecisionDx-Melanoma compare to other GEP tests?
Other GEP tests report a 74% recurrence-free survival (RFS) rate in their high-risk category, while reporting an 89% RFS in their low-risk population9. Notably, an 89% RFS is a threshold many oncologists would still consider the risk significant enough to warrant adjuvant therapy.10
Expert Insights: Discover why Shannon Trotter, DO, FAOCD, FAAD, chooses DecisionDx-Melanoma and how it enhances patient management
Castle Biosciences: The leader in melanoma prognostic testing leader with independent, robust validation and real-world results

50+
peer-reviewed, pubished studies including prospective studies and two meta-analyses

200k+
patients with a clinical DecisionDx-Melanoma order from ~13,000 clinicians

Medicare+
covered by Medicare and multiple private insurers with an industry-leading patient assistance program
*Numbers as of May 2025
Ensure you’re using DecisionDx-Melanoma—the most clinically evidenced GEP test—to guide management decisions for your melanoma patients


.svg)
References
- Bailey et al. JCO PO. 2023
- Whitman et al. JCO PO. 2021
- Jarell et al. JAAD. 2022
- Prieto et al. Cancer Diagnosis & Prognosis. 2025
- Gerami et al. Clin Cancer Res. 2015
- Gerami et al. JAAD. 2015
- Zager et al. BMC Cancer. 2018
- Gastman et al. JAAD. 2019
- Eggermont et al. EJC. 2020
- Fastner et al. Cancer Medicine. 2023
