Informing Decisions, Improving Outcomes
Improves outcomes by guiding personalized, risk-aligned management decisions
DecisionDx-Melanoma is a gene expression profile test that provides comprehensive, personalized results to guide risk-aligned management decisions for patients with stage I-III cutaneous melanoma. By harnessing the genetic information within a patient’s tumor biology and incorporating traditional clinicopathologic features, DecisionDx-Melanoma helps to stratify patient risk and provide personalized results regarding a patient's risk of recurrence and/or metastasis and likelihood of sentinel lymph node positivity. DecisionDx-Melanoma can help to improve prognostic accuracy, better inform management decisions and positively impact outcomes – it is the only melanoma prognostic test associated with improved survival.
The largest real-world study of gene expression profile testing in melanoma
Recent data from an ongoing collaboration with the National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER) Program Registries show that testing with DecisionDx-Melanoma was associated with lower melanoma-specific and overall mortality relative to untested patients. In the study, DecisionDx-Melanoma provided significant and independent risk stratification of patients with cutaneous melanoma, beyond American Joint Committee on Cancer Eighth Edition (AJCC8) stage, which may help inform more personalized patient management decisions (Bailey et al, 2023).
Comprehensive results, integrating clinicopathologic features with GEP, to inform clinical management decisions
DecisionDx-Melanoma integrates a patient’s tumor biology and clinicopathologic features to provide precise risk prediction to answer two key clinical questions – the likelihood of sentinel lymph node positivity and risk of recurrence. These personalized results help to better inform management decisions.
Personalized risk of sentinel lymph node positivity
Sentinel lymph node biopsy (SLNB) is used to identify patients whose cutaneous melanoma has progressed (i.e., spread to a nearby lymph node), and who are therefore in need of additional therapeutic intervention.
The 31-GEP score is integrated with a patient’s clinicopathologic factors including Breslow thickness, ulceration, mitotic rate, and age to provide an individualized risk of sentinel lymph node (SLN) positivity.
This personalized result helps physicians make management decisions, including patients with <5% risk who may be able to safely forego SLNB.
Personalized risk of recurrence
DecisionDx-Melanoma provides a personalized risk of recurrence for melanoma-specific survival, recurrence-free survival, and distant metastasis-free survival. (MSS, RFS, DMFS)
The 31-GEP score is now integrated with a patient’s clinicopathologic factors including Breslow thickness, ulceration, mitotic rate, SLN status, age, and tumor location to provide an individualized risk of tumor recurrence.
This personalized result helps physicians make management decisions, including clinic visits, specialist referrals, and treatment.
Addresses melanoma staging contradictions
Patients diagnosed with the same stage of melanoma can have very different tumor biology, and therefore, very different risks for developing recurrence and metastases. Traditional staging can fail to identify patients with aggressive tumor biology – misidentifying patients who have high-risk tumor biology as lower risk at the time of diagnosis. DecisionDx-Melanoma provides personalized prognostic information, guiding individualized management care decisions, such as increased surveillance, which have been shown to lead to earlier detection and, in turn, improved outcomes.
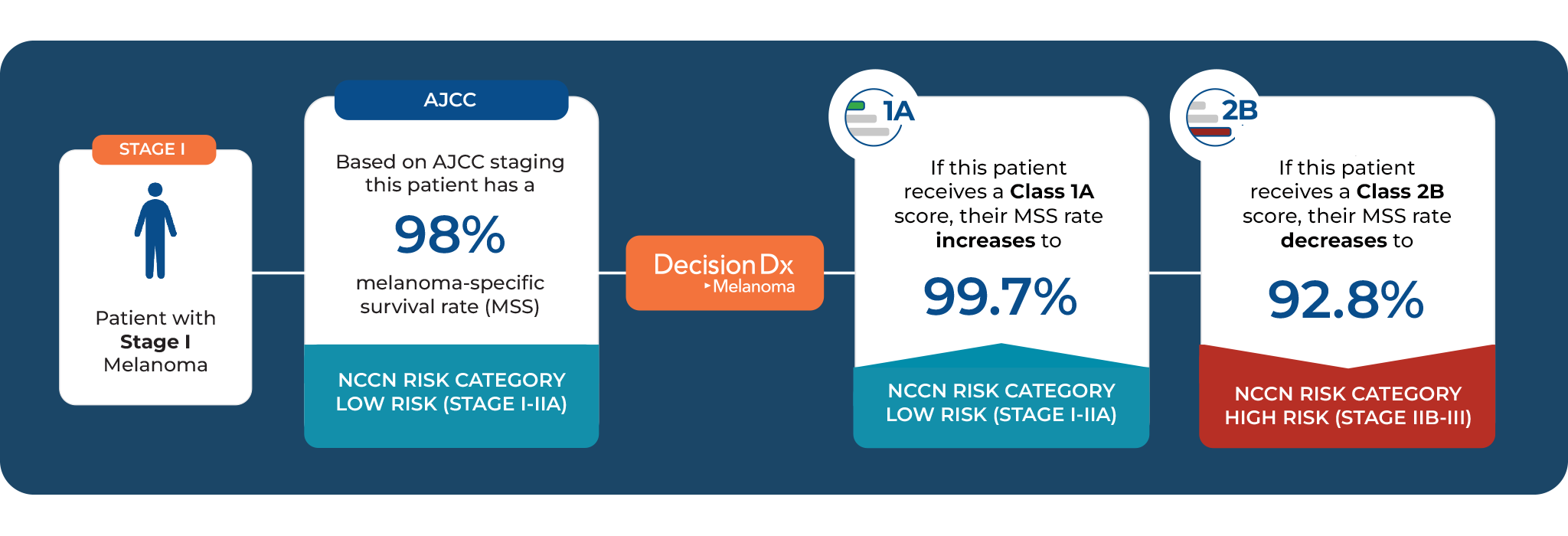
DecisionDx-Melanoma provides more specific, personalized, risk estimates compared to staging alone
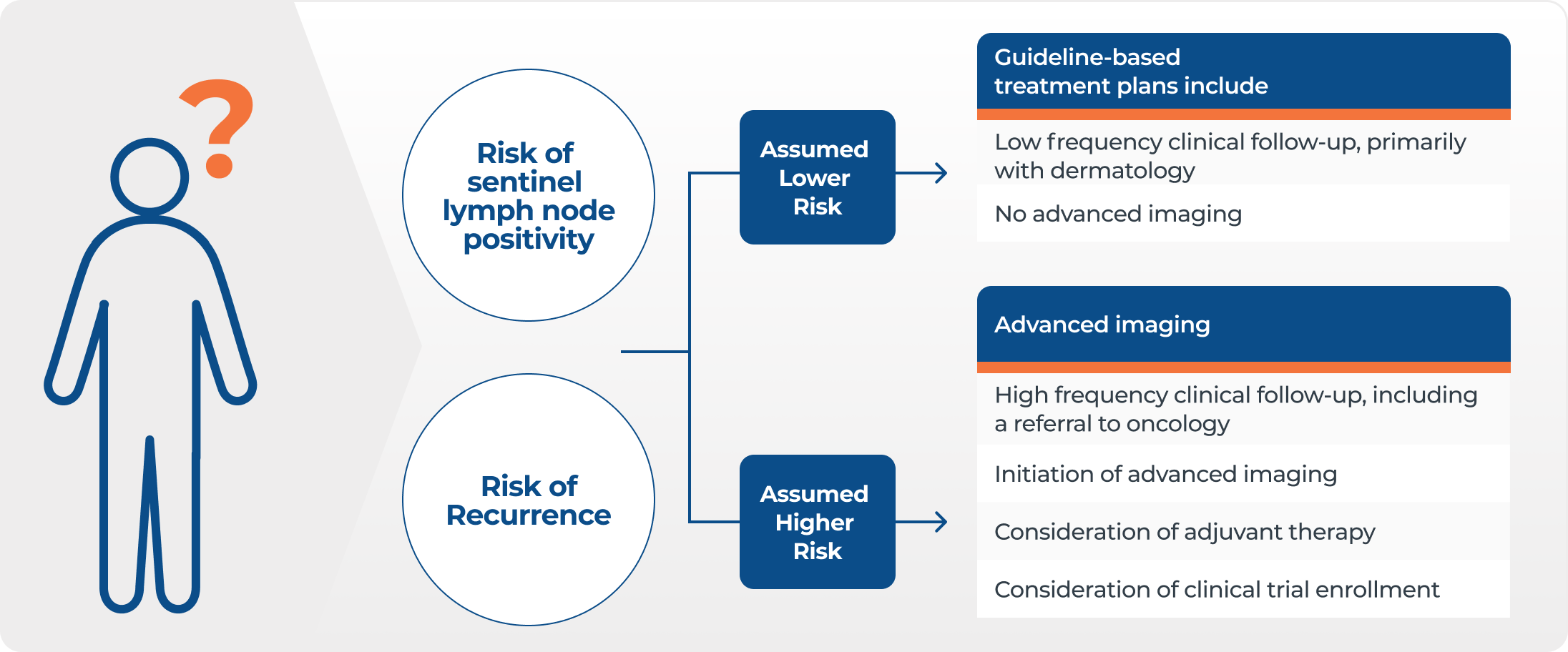
Guides risk-aligned patient management
Across various studies, patients tested with DecisionDx-Melanoma have been shown to have better outcomes than those who are not tested. This is likely due to changes in management based on the additional prognostic information DecisionDx-Melanoma testing provides. In fact, 1 in 2 patients tested had changes in their management plans including frequency of follow-up, lab work and surveillance monitoring. (Dillon et al, 2022)
DecisionDx-Melanoma has demonstrated clinical impact and utility.