


TissueCypher Barrett’s esophagus assay impacts clinical decisions in the management of patients with Barrett’s esophagus
David L. Diehl1, Harshit S. Khara1, Nasir Akhtar1, Rebecca J. Critchley-Thorne2
1 Department of Gastroenterology, Geisinger Health System, Danville, Pennsylvania, United States; 2 Research and Development, Castle Biosciences, Inc., Pittsburgh, PA, USA
TissueCypher has been shown to risk-stratify Barrett’s esophagus patients in five clinical validation studies. Given that clinical validation, this study was conducted in conjunction with Geisinger Medical Center to assess the impact of TissueCypher on clinicians’ management decisions. This was a prospective study that evaluated patients undergoing endoscopic surveillance from June 2016 to April 2020. A pre-TissueCypher management plan survey was used to document the initial treatment plan based on clinical and pathology information, and a post-TissueCypher management plan survey was used to document changes to the initial plan once the TissueCypher results were reviewed by the physicians.
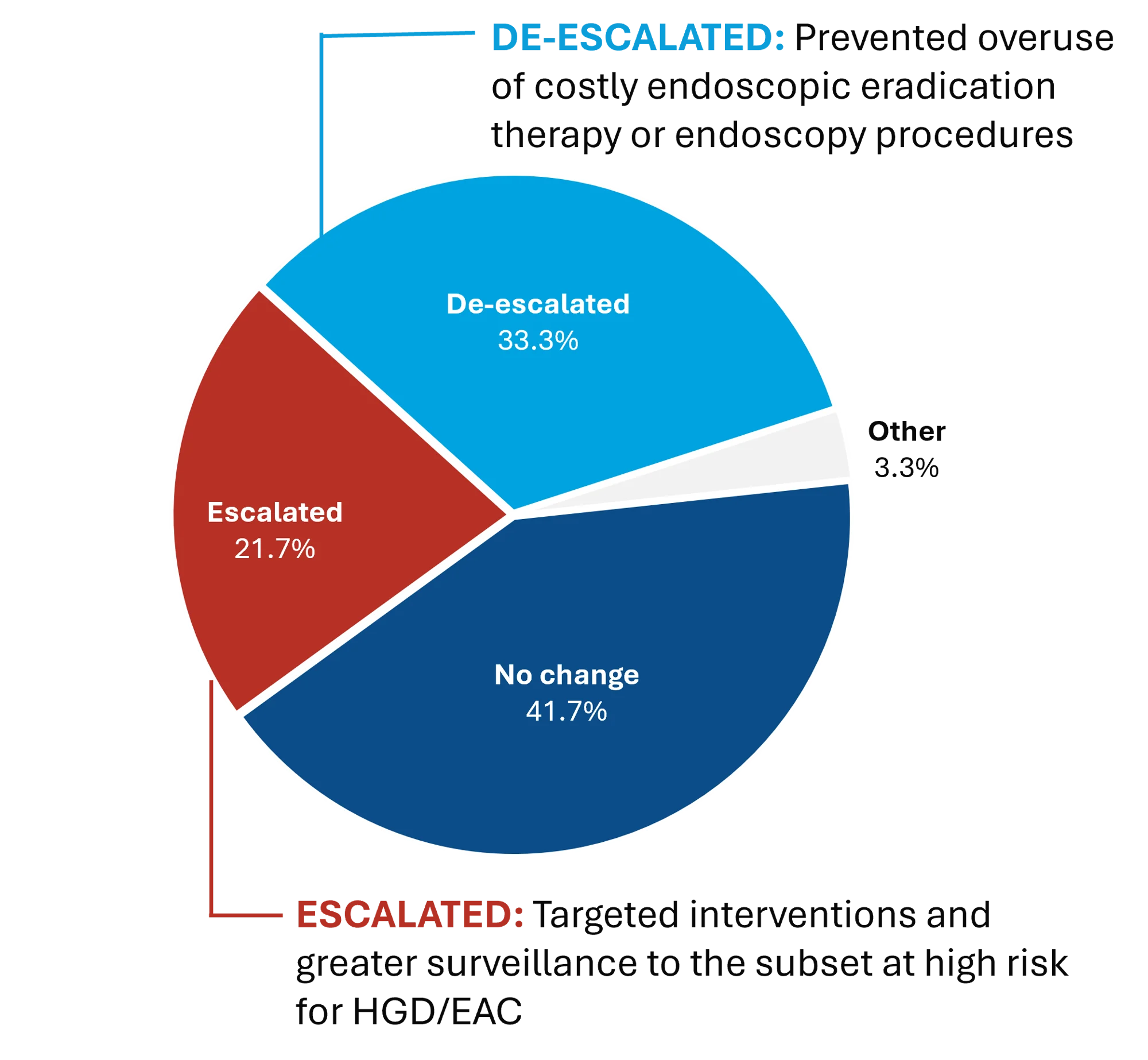
The study revealed that results from the TissueCypher Barrett’s Esophagus test changed the management plan for 55% of patients in the study.
- In 21.7% of patients, the test upstaged the management approach, resulting in endoscopic eradication therapy (EET) or shorter interval surveillance.
- The test downstaged the management approach in 33.3% of patients, resulting in surveillance rather than EET or longer surveillance intervals.
In the subset of patients whose management plan was changed, upstaging was associated with a high-risk TissueCypher result, and downstaging was associated with a low-risk result (p < 0.0001).
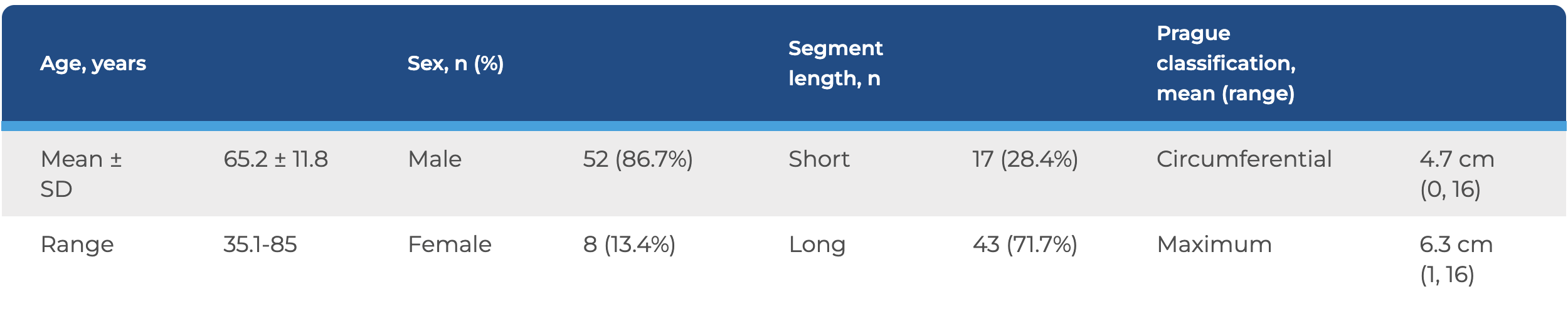
In total, 60 patients were included in the study. Their ages ranged from 35 to 80 years old, 87% were male, and 72% had long-segment Barrett’s esophagus with a wide range of Prague classifications.
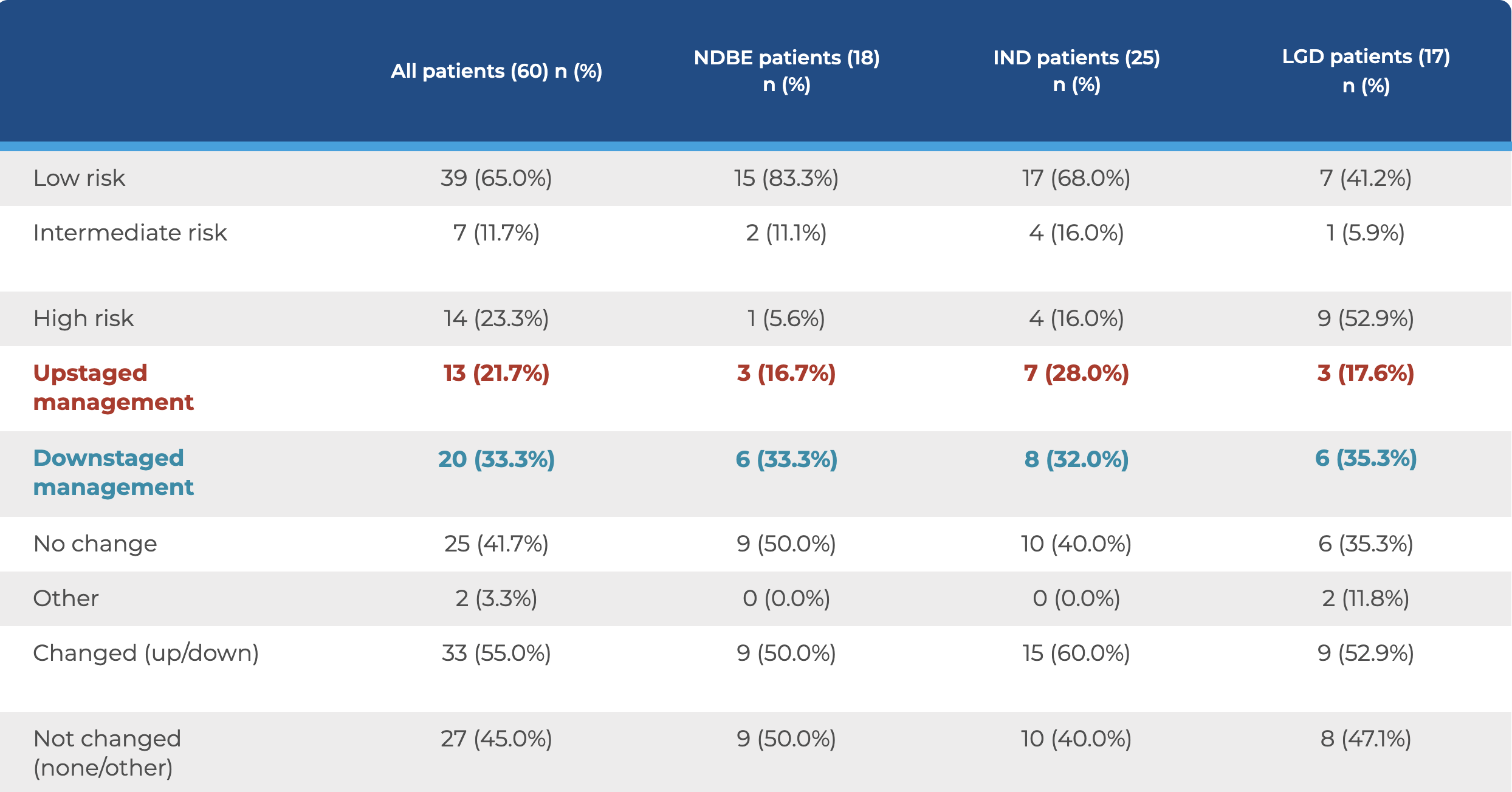
TissueCypher testing was performed on Barrett’s esophagus tissue biopsies from the 60 patients who had received an expert pathology diagnosis of non-dysplastic Barrett’s esophagus (NDBE), indefinite for dysplasia (IND), or low-grade dysplasia (LGD). The test results provided clinicians with a risk score (0–10), a risk class (high, intermediate, or low), and a 5-year probability of progressing to high-grade dysplasia (HGD) or esophageal adenocarcinoma (EAC). The clinicians evaluated this information along with clinicopathologic risk factors to decide if it was appropriate to upstage, downstage, or maintain the original care plan for each patient. In the majority of cases, across all histology grades, the physicians chose to adjust their care plan.
A high-risk score from TissueCypher provides an additional, independent risk factor for clinicians to consider when determining appropriate management. In the video below, Dr. Khara discusses a a case subsequent to this study where he used a TissueCypher score to upstage his management
A low-risk score from TissueCypher provides value in the form of peace of mind for clinicians and their patients. In the case discussion below, Dr. Khara explains how he used a low-risk score in one of his subsequent cases after this study.











