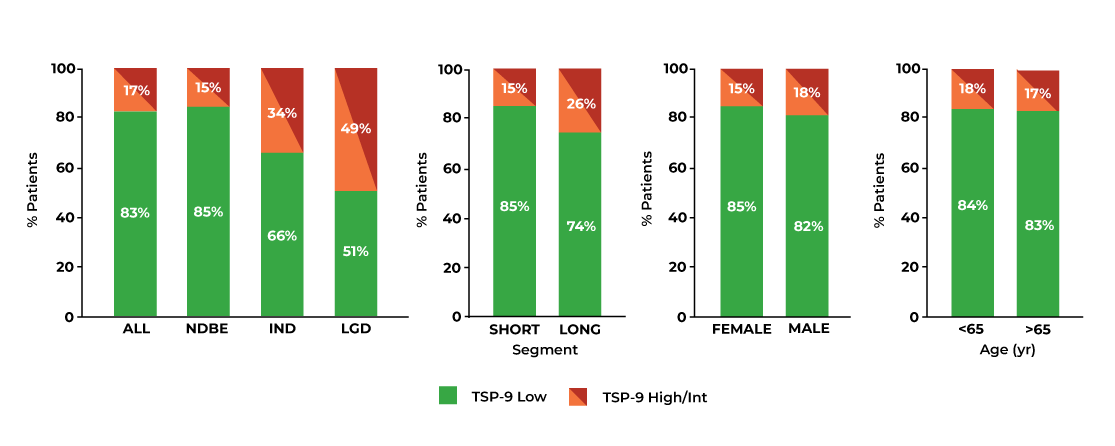
The Tissue Systems Pathology test provides clinically actionable risk stratification in patients with Barrett’s esophagus: A multicenter U.S. clinical experience
Villa NA, Ordonez-Castellanos M, Yodice M, et al. The tissue systems pathology test objectively risk-stratifies patients with Barrett’s esophagus results from a multicenter US clinical experience study. J Clin Gastroenterol. 2024 Jul 2. doi: 10.1097/MCG.0000000000002040. Online ahead of print.
Want to learn more about
DecisionDx-Melanoma?