


The QURE clinical utility study provides support for TissueCypher's ability to significantly improve physician adherence to clinical guidelines for Barrett's esophagus (BE)
Peabody JW, Cruz JDC, Ganesan D, et al. A randomized controlled study on clinical adherence to evidence-based guidelines in the management of simulated patients with Barrett’s esophagus and the clinical utility of a tissue systems pathology test: results from Q-TAB. Clin Transl Gastroenterol. 2023. https://doi.org/10.14309/ctg.0000000000000644
TissueCypher has the potential to significantly improve clinical care decisions
Findings from this study demonstrate that TissueCypher has the potential to significantly improve the quality of clinical care decisions for BE management, enabling risk aligned care decisions that may lead to better patient health outcomes. This study provides support for TissueCypher's ability to:
- Optimize adherence to clinical guidelines
- Improve accuracy of patient risk assessment
- Increase appropriate recommendations for endoscopic eradication therapy (EET).
Studies have shown that in practice, clinician adherence to society guidelines is limited.1 This is largely because BE is a disease state with risk factors that are often incongruent, and diagnosis and treatment plans rely on subjectivity of pathologic analysis.
This study aimed to evaluate the impact of TissueCypher on adherence to guidelines as well as the accuracy of risk-aligned management decisions that lead to improved patient health outcomes.
Board-certified gastroenterologists or GI surgeons (n=259) were assigned to three groups: Control, Intervention 1, Intervention 2, and were asked to evaluate Clinical Performance and Value (CPV) vignettes, which are realistic simulations of patient cases in which physicians proceeded through domains of a clinical encounter and made management decisions. The simulated patients represented typical Barrett’s patients with a range of clinical factors and TissueCypher risk results. Next, the physicians in the Intervention 1 and 2 group received education on TissueCypher testing after which all three groups were asked to evaluate a new set of CPVs. The quality of the management decisions that were made by the physicians were scored based on adherence to society guidelines by two trained, expert physicians working independently, and any disagreement was adjudicated by a third physician.
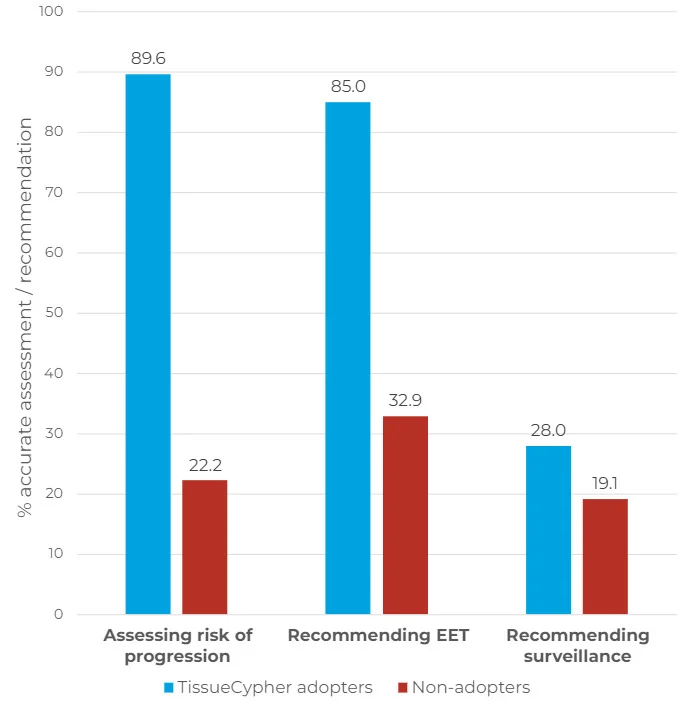
- The clinicians that chose to order TissueCypher were significantly better at identifying the risk of progression to HGD/EAC (Figure 1) – 89.6% for test "adopters" vs. 22.2% for "non-adopters" (p < 0.001).
- 85% of TissueCypher adopters appropriately ordered EET given the risk of progression, compared to only 32.9% of non-adopters (p < 0.001).
- When physicians were given the option to order the test in Intervention 2, it was ordered in 21.9% of cases, with no significant difference in ordering by case type, variant, specialty, practice type, or practice setting. This is consistent with the average adoption rate of 25% for new diagnostic technologies².
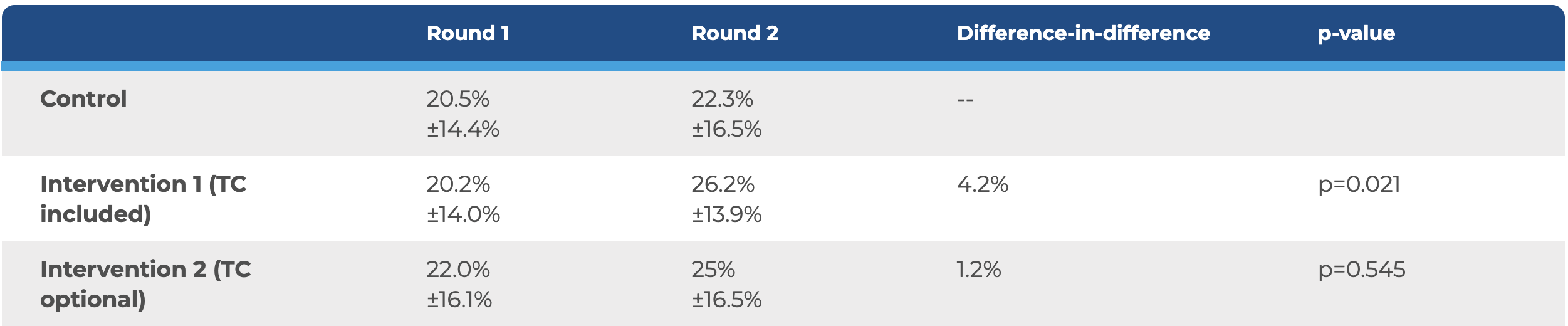
- Before TissueCypher was introduced, no significant difference was found for the percent appropriate diagnosis and treatment plans (DxTx) across each arm of the study.
- Following incorporation of TissueCypher , Intervention 1 showed a significant difference-in-difference improvement of +4.2% (p=0.021) across all variants.
- The most dramatic increase in performance was seen for simulated patients with low clinical risk who received a TissueCypher high-risk result. Physicians more accurately assessed the risk of disease progression, showing a difference-in-difference score of +65.7 (p<0.001).
- For cases requiring annual endoscopic surveillance, significant improvement was found in adherence for Intervention 1, with a difference-in-difference of +18.5% (p=0.019).
Evidence-based diagnosis + treatment guideline adherence by study arm for all cases
Financial support: This study was funded by Cernostics, Inc. Pittsburgh, Pennsylvania, USA, a wholly owned subsidiary of Castle Biosciences, Inc.
IRB Approval Statement: This study was approved by the Advarra Institutional Review Board, Columbia, MD, in accordance with ethical standards, and is listed on clinicaltrials.gov (NCT05200325). Informed consent was obtained from all participants through an online voluntary consent process.
Clinical Trials Registry: ClinicalTrials.gov (NCT05200325).
References
1 Cruz JD, Paculdo D, Ganesan D, et al. Clinical variation in surveillance and management of Barrett’s esophagus: A cross-sectional study of gastroenterologists and gastrointestinal surgeons. Medicine (Baltimore) 2022;101(51):e32187.
2 Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011; 104: 510.











