Explore the clinical studies and scientific evidence validating DecisionDx-UM
The DecisionDx-UM gene expression profile (GEP) test is a robust multianalyte assay that accurately identifies which patients are at high and low risk of developing metastatic disease within five years. The test, along with specimen collection techniques, has been validated on both fresh-frozen samples obtained from fine-needle aspirate biopsy procedures (FNAB), and on archival tissue derived from formalin-fixed, paraffin-embedded (FFPE) samples.
Clinically, the test has been clinically validated and is no longer considered experimental or investigational. Many validation and performance studies have been conducted to confirm the clinical accuracy of DecisionDx-UM and include multiple independent retrospective and prospective sample sets.
Scientific references for DecisionDx-UM

- Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010;12(4):461-468. doi: 10.2353/jmoldx.2010.090220
- Plasseraud KM, Wilkinson JK, Oelschlager KM, et al. Gene expression profiling in uveal melanoma: technical reliability and correlation of molecular class with pathologic characteristics. Diagn Pathol. 2017;12(1):59. doi: 10.1186/s13000-017-0650-3
- Klofas LK, Bogan CM, Coogan AC, Schultenover SJ, Weiss VL, Daniels AB. Instrument gauge and type in uveal melanoma fine needle biopsy: implications for diagnostic yield and molecular prognostication. Am J Ophthalmol. 2021;221:83-90. doi: 10.1016/j.ajo.2020.08.014

- Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596-1603. doi: 10.1016/j.ophtha.2012.02.017
- Gill HS, Char DH. Uveal melanoma prognostication: from lesion size and cell type to molecular class. Can J Ophthalmol. 2012;47(3):246-253. doi: 10.1016/j.jcjo.2012.03.038
- Chappell MC, Char DH, Cole TB, et al. Uveal melanoma: molecular pattern, clinical features, and radiation response. Am J Ophthalmol. 2012;154(2):227-232.e2. doi: 10.1016/j.ajo.2012.02.022
- Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):131-135. doi: 10.1007/s00417-013-2515-0
- Corrêa ZM, Augsburger JJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am J Ophthalmol. 2016;162:20-27.e1. doi: 10.1016/j.ajo.2015.11.019
- Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134(7):728-733. doi: 10.1001/jamaophthalmol.2016.0903
- Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol. 2016;134(7):734-740. doi: 10.1001/jamaophthalmol.2016.0913
- Plasseraud KM, Cook RW, Tsai T, et al. Clinical performance and management outcomes with the DecisionDx-UM gene expression profile test in a prospective multicenter study. J Oncol. 2016;2016:5325762. doi: 10.1155/2016/5325762
- Demirci H, Niziol LM, Ozkurt Z, et al. Do largest basal tumor diameter and the American Joint Committee on Cancer’s cancer staging influence prognostication by gene expression profiling in choroidal melanoma. Am J Ophthalmol. 2018;195:83-92. doi: 10.1016/j.ajo.2018.07.033
- Cai L, Paez-Escamilla M, Walter SD, et al. Gene expression profiling and PRAME status versus tumor-node-metastasis staging for prognostication in uveal melanoma. Am J Ophthalmol. 2018;195:154-160. doi: 10.1016/j.ajo.2018.07.045
- Afshar AR, Damato BE, Stewart JM, et al. Next-generation sequencing of uveal melanoma for detection of genetic alterations predicting metastasis. Transl Vis Sci Technol. 2019;8(2):18. doi: 10.1167/tvst.8.2.18
- Aaberg TM, Covington KR, Tsai T, et al. Gene expression profiling in uveal melanoma: five-year prospective outcomes and meta-analysis. Ocul Oncol Pathol. 2020;6(5):360-367. doi: 10.1159/000508382
- Binkley EM, Bena JF, Davanzo JM, Hinz C, Boldt HC, Singh AD. Gene expression profiling prognostication of posterior uveal melanoma: does size matter? Ophthalmol Retina. 2020;4(6):620-629. doi: 10.1016/j.oret.2019.12.020
- Roelofs KA, Grewal P, Lapere S, Larocque M, Murtha A, Weis E. Optimising prediction of early metastasis-free survival in uveal melanoma using a four-category model incorporating gene expression profile and tumour size. Br J Ophthalmol. 2022;106(5):724-730. doi: 10.1136/bjophthalmol-2020-317714
- Stacey AW, Dedania VS, Materin M, Demirci H. Improved prognostic precision in uveal melanoma through a combined score of clinical stage and molecular prognostication. Ocul Oncol Pathol. 2022;8(1):35-41. doi: 10.1159/000520218
- Kheir WJ, Stinnett SS, Meltsner S, et al. Preliminary results of uveal melanoma treated with iodine-125 plaques: analysis of disease control and visual outcomes with 63 Gy to the target volume. Adv Radiat Oncol. 2022;7(2):100869. doi: 10.1016/j.adro.2021.100869
- Wong AJ, Teh BS, Nguyen BT, et al. Three-year outcomes of uveal melanoma treated with intra-operative ultrasound-guided iodine-125 brachytherapy using custom-built eye plaques. J Contemp Brachytherapy. 2022;14(2):130-139. doi: 10.5114/jcb.2022.115379
- Singh AD, Binkley EM, Wrenn JM, Bena JF, Hinz C, Boldt HC. Predicted vs observed metastasis-free survival in individuals with uveal melanoma. JAMA Ophthalmol. 2022;140(9):847-854. doi: 10.1001/jamaophthalmol.2022.2623
- Harbour JW, Correa ZM, Schefler AC, et al. 15-Gene expression profile and PRAME as integrated prognostic test for uveal melanoma: First report of collaborative ocular oncology group study no. 2 (COOG2.1). J Clin Oncol. 2024;JCO2400447. doi: 10.1200/JCO.24.00447

- Aaberg TM, Cook RW, Oelschlager K, Maetzold D, Rao PK, Mason JO. Current clinical practice: differential management of uveal melanoma in the era of molecular tumor analyses. Clin Ophthalmol. 2014;8:2449-2460. doi: 10.2147/OPTH.S70839
- Plasseraud KM, Cook RW, Tsai T, et al. Clinical performance and management outcomes with the DecisionDx-UM gene expression profile test in a prospective multicenter study. J Oncol. 2016;2016:5325762. doi: 10.1155/2016/5325762
- Davanzo JM, Binkley EM, Bena JF, Singh AD. Risk-stratified systemic surveillance in uveal melanoma. Br J Ophthalmol. 2019;103(12):1868-1871. doi: 10.1136/bjophthalmol-2018-313569
- Schefler AC, Skalet A, Oliver SC, et al. Prospective evaluation of risk-appropriate management of uveal melanoma patients informed by gene expression profiling. Melanoma Manag. 2020;7(1):MMT37. doi: 10.2217/mmt-2020-0001
- Aaberg TM, Covington KR, Tsai T, et al. Gene expression profiling in uveal melanoma: five-year prospective outcomes and meta-analysis. Ocul Oncol Pathol. 2020;6(5):360-367. doi: 10.1159/000508382
- Yesiltas YS, Zabor EC, Wrenn J, et al. Surveillance for metastasis in high-risk uveal melanoma patients: standard versus enhanced protocols. Cancers. 2023;15(20):5025. doi: 10.3390/cancers15205025
Summary of clinical validity and performance studies with reported outcomes
Summary of studies comparing DecisionDx-UM to clinicopathologic and genetic features
| Study | n | Factors examined | Comparison to other prognostic features |
|---|---|---|---|
| Onken et al., 2012 (COOG) | 446 | Age, ciliary body involvement, tumor diameter, tumor thickness, cell type, chromosome 3 status | DecisionDx-UM was the only significant independent predictor of metastasis in multivariate analysis (p=0.006) |
| Chappell et al., 2012 | 187 | Tumor size, cell type, age | DecisionDx-UM was the only significant predictor of metastasis-free survival and disease-specific survival in multivariate analysis (p<0.0001 for both) |
| Gill & Char, 2012 | meta-analysis | Clinical, cytogenetic, histologic features | Molecular class has the strongest predictive value for metastasis and mortality |
| Correa & Augsburger, 2014 | 158 | Cytopathology | DecisionDx-UM was superior to cytologic classification for predicting metastasis and death |
| Correa & Augsburger, 2016 | 299 | Age, gender, ciliary body involvement, largest basal diameter, thickness, cell type | DecisionDx-UM was the strongest prognostic factor for melanoma-specific mortality (p=0.0019) in multivariate analysis |
| Walter et al., 2016 | 580 (346 not in COOG) | Age, ciliary body involvement, largest basal diameter, thickness, cell type | DecisionDx-UM was the strongest prognostic factor for PFS and the only significant prognostic factor for OS in multivariate analysis (p<0.001 for both) |
| Plasseraud et al., 2016 | 70 | Age, largest basal diameter, ciliary body involvement, tumor thickness | DecisionDx-UM was the strongest prognostic factor for metastasis in multivariate and Kaplan-Meier analyses (p=0.016) |
| Decatur et al., 2016 | 81 | GNAQ, GNA11, BAP1, SF3B1, EIF1AX mutations; age, gender, ciliary body involvement, cell type, extraocular extension, tumor thickness, largest basal diameter | DecisionDx-UM was the strongest predictor of metastasis and melanoma-specific mortality (p<0.001 for both) in multivariate analysis |
| Aaberg et al., 2020 | 89 | Age, largest basal diameter, ciliary body involvement, tumor thickness | DecisionDx-UM was the strongest prognostic factor for metastasis in multivariate analysis (p<0.0001) |
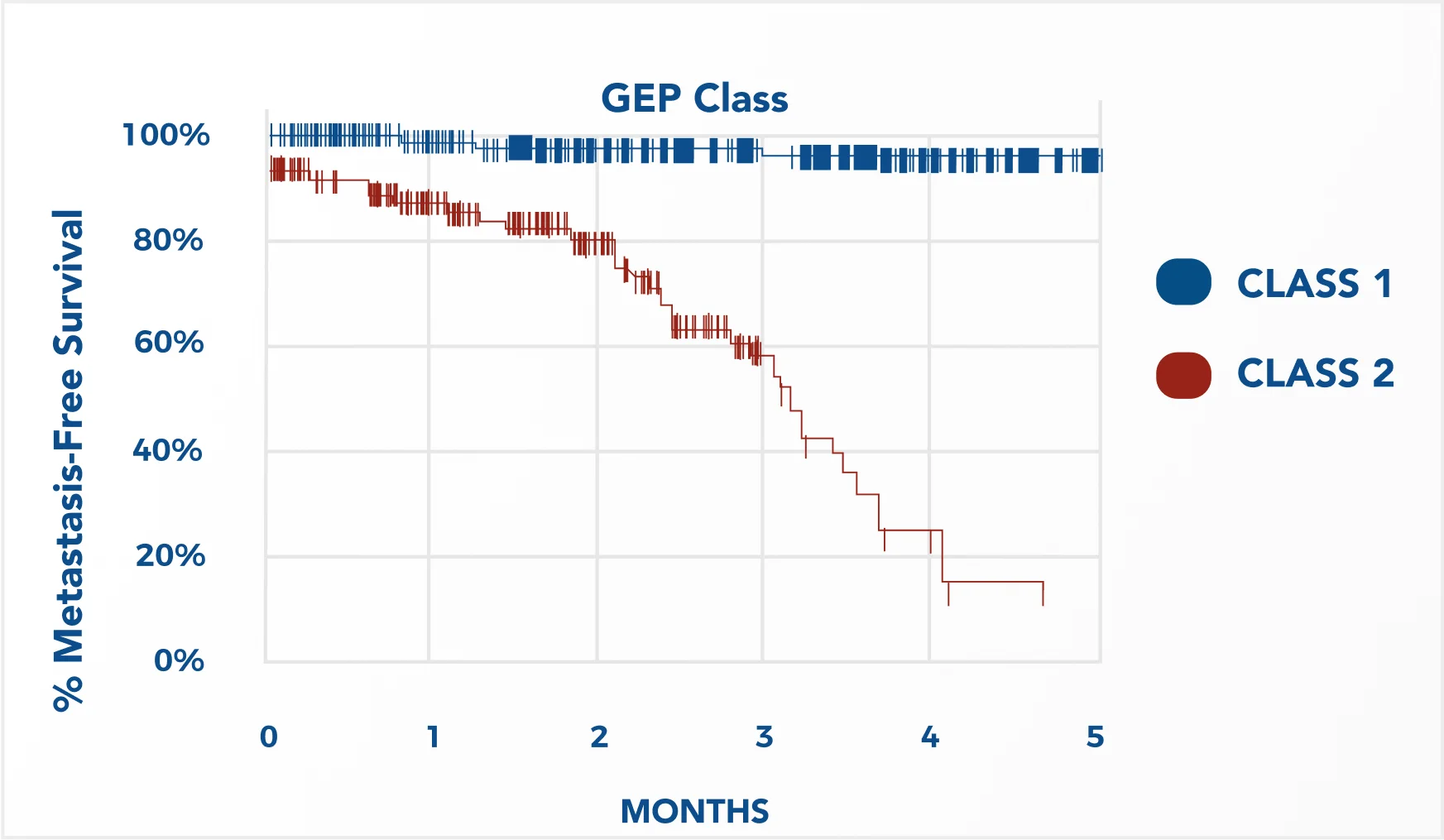
Study highlight: COOG study finds DecisionDx-UM robust and highly accurate
The first prospective validation study of DecisionDx-UM was a 5-year prospective, multicenter, 494-patient study by the Collaborative Ocular Oncology Group (COOG), published in Ophthalmology (Onken et al, 2012). This study compared DecisionDx-UM to all clinical and pathologic prognostic factors and found it to be superior to, and independent of, all such factors (eg, tumor diameter, tumor thickness, patient age, histopathology involvement, cytopathology and chromosome 3 status).
Consistent with prior retrospective and prospective single-center studies, the COOG study found the DecisionDx-UM test to be accurate in differentiating between low-risk (Class 1) tumors and high-risk (Class 2) tumors (log-rank test, p<10e-4). The Kaplan Meier plot below shows the percent of Class 1 and Class 2 patients who remained metastasis-free over 50 months of follow-up. Additional data from this study, including multivariate analyses, can be found in comparison to other prognostic markers.
Meets highest levels of validation
DecisionDx-UM is the only prognostic test for uveal melanoma that has been clinically validated by an independent prospective, multi-center study, as well as multiple retrospective and prospective single-center studies. Twenty-five peer-reviewed scientific publications, including analytical validation, clinical validation, clinical utility and patient perspective studies, support the clinical use of DecisionDx-UM.

Analytical validity
- CLIA Lab Analytical Validity Study
- CAP Accreditation
- NYS DoH Approval
- State Licenses - CA, FL, MD, NY, PA, RI
- Onken et al. J Mol Diagn. 2010
- Plasseraud et al. Diagn Pathol. 2017
- Klofas et al. Am J Ophthalmol. 2020

Clinical validity
- Onken et al. Ophthalmology 2012
- Gill and Char. Can J Ophthalmol. 2012
- Chappell et al. Am J Ophthalmol. 2012
- Correa et al. Graef Arch Clin Exp Ophthalmol. 2014
- Correa et al. Am J Ophthalmol. 2016
- Decatur et al. JAMA Ophthalmol. 2016
- Walter et al. JAMA Ophthalmol. 2016
- Plasseraud et al. J Oncol. 2016
- Demirci et al. Am J Ophthalmol. 2018
- Cai et al. Am J Ophthalmol. 2018
- Afshar et al. Transl Vis Sci Technol. 2019
- Aaberg et al. Ocul Oncol Pathol. 2020
- Binkley et al. Ophthalmol Retina. 2020

Clinical validity
- Aaberg et al. Clin Ophthalmol. 2014
- Plasseraud et al. J Oncol. 2016
- Davanzo et al. Br J Ophthalmol. 2019
- Schefler et al. Melanoma Manag. 2020
- Aaberg et al. Ocul Oncol Pathol. 2020






