

Enables accurate determination of metastatic risk in uveal melanoma
Discover how DecisionDx-UM offers personalized prognostic insights for uveal melanoma
The DecisionDx-UM gene expression profile (GEP) test enables accurate determination of metastatic risk in uveal melanoma. The test identifies the molecular signature of an individual’s tumor and its likelihood of metastasis within 5 years. Specifically, the assay determines the activity or “expression” of 15 genes which indicate a patient’s individual risk, or class.
Primary clinical issue: developing risk-appropriate treatment plans for individual patients
While uveal melanoma is the most common intraocular tumor in adults, it is a rare cancer with an annual U.S. incidence of approximately 2,000.
Despite a 93% to 98% primary tumor “cure” rate, up to 50% of all patients will ultimately develop metastatic disease, primarily to the liver. However, less than 4% of patients present with metastatic disease at the time of diagnosis.
Following treatment for the primary eye tumor, the main clinical issue in uveal melanoma is determining a patient’s metastatic risk so that a risk-appropriate surveillance and management plan can be implemented.
The need for DecisionDx-UM
Traditional staging does not adequately stratify patient for risk-based management decisions.
- 12% 15 year mortality in lowest-stage tumors
- Historically, most physicians performed intensive surveillance on their UM patients
- Molecular classification with GEP has changed the paradigm by grouping patients into clinically actionable risk groups
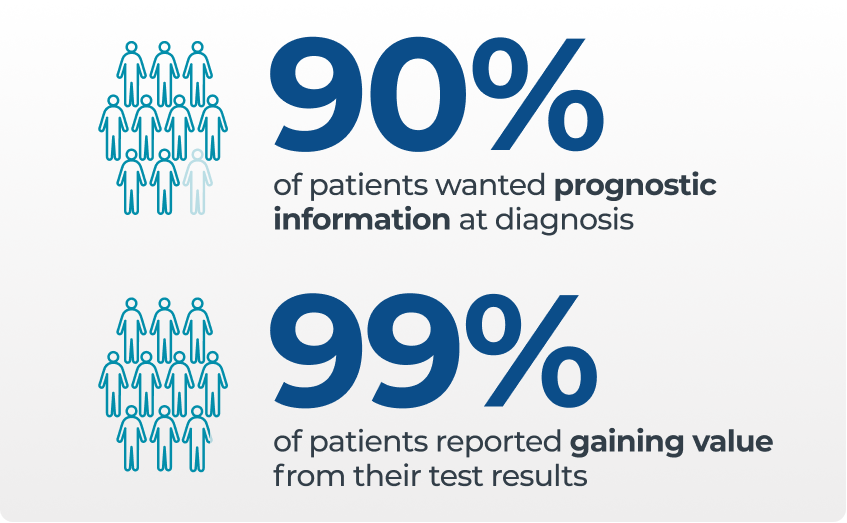
- Patients want to know their risk
Changing how uveal melanoma is managed
DecisionDx-UM identifies which patients are at low or high risk for the progression of their disease so their physicians can appropriately adjust the level of care provided.
Since its introduction in late 2009, it has been adopted as the standard of care by the majority of ocular oncology specialists in the U.S. to inform risk-appropriate clinical care decisions.






