MyPath Melanoma ordering process & results
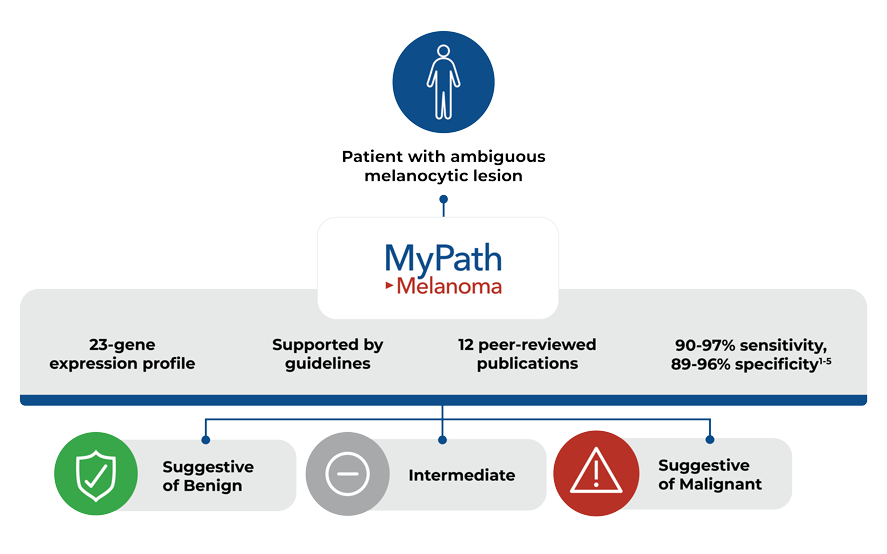
MyPath Melanoma is intended to aid in the characterization of primary cutaneous melanocytic lesions with uncertain malignant potential. Test results should be interpreted in the context of other clinical, laboratory and histological information.
Ordering MyPath Melanoma

Ordering MyPath Melanoma
The test can be requested via Castle’s secure online portal or by completing a test request form. Click below to download a request form or register for Castle’s secure portal.

Testing process
Castle’s Clinical Services team coordinates the collection and shipping of tissue blocks or slides directly from the pathology lab provided on the requisition form. The MyPath Melanoma test uses the same tissue from the original biopsy or excision procedure, avoiding additional procedures. This assay requires 10% tumor content.

Report results
The MyPath test result is reported as one of three categories: suggestive of benign, intermediate, or suggestive of malignant. A sample test report is available at the link below. Test results are available via our secure online portal within 3-5 business days from sample receipt.

Patient access
Castle Biosciences is committed to providing high-quality molecular testing to all patients. We do not want financial concerns to be a barrier to patients accessing critical healthcare information. We intend to work with all insurance providers, including Medicare, Medicaid, commercial insurers, and Veterans Affairs (VA), to secure payment coverage for our testing.
For those who may need additional assistance with their Castle test, we offer a comprehensive patient assistance program to all patients.
If you have any questions, please contact us by calling
866-788-9007, option 3 or send an email.
Validated to classify ambiguous melanocytic lesions as suggestive of benign or suggestive of malignant
[.text-size-citation]1. Clarke L, Warf M, Flake D, et al. J Cutan Pathol. 2015;42:244-52.[.text-size-citation]
[.text-size-citation]2. Clarke L, Pimental J, Zalaznick H, et al. Hum Pathol. 2017;70:113-20.[.text-size-citation]
[.text-size-citation]3. Ko J, Matharoo-Ball B, Billings S, et al. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-13.[.text-size-citation]
[.text-size-citation]4. Ko J, Clarke L, Minca E, et al. Hum Pathol. 2019;86:213-21.[.text-size-citation]
[.text-size-citation]5. Clarke L, Mabey B, Flake D, et al. Per Med. 2020;17:361-71.[.text-size-citation]
Actionable results for clinicians and pathologists
Increased clarity in pathology reports
More informed patient management plans
Improved diagnostic and clinical confidence