Informs risk appropriate patient management
DecisionDx-SCC is a qRT-PCR assay of 6 control and 34 discriminant genes (40 in total) that uses a validated, proprietary neural network algorithm comprised of two gene expression signatures to classify patients into risk categories. The more personalized risk stratification or prognosis helps guide the most appropriate and individualized care for patients with SCC.
More biological insight, more appropriate patient management

Assesses
40 genes
34 discriminating and 6 endogenous control genes
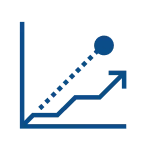
Provides
Precise, personalized risk of metastasis and likelihood to benefit from adjuvant radiation therapy to better inform patient management decisions

Informs
Patient management decisions including appropriateness of adjuvant radiation therapy, imaging, and frequency of surveillance
DecisionDx-SCC testing process
DecisionDx-SCC is performed in Castle Biosciences’ CLIA certified, CAP-accredited and NY state approved laboratory using formalin-fixed, paraffin embedded (FFPE) primary tumor tissue from either a biopsy or excision.
Tissue biopsy submitted
Tissue blocks or slides from the primary tumor tissue biopsies are sent to our laboratory for testing.

RNA/DNA preparation
Once tissue is received in our laboratory, RNA is isolated from the tissue and converted to cDNA. The cDNA for each of the 40 genes is amplified and the gene signature recorded.
Signature interpretation
Using an independently validated algorithm, the individual patient's gene expression signature is assessed and a class score is provided.

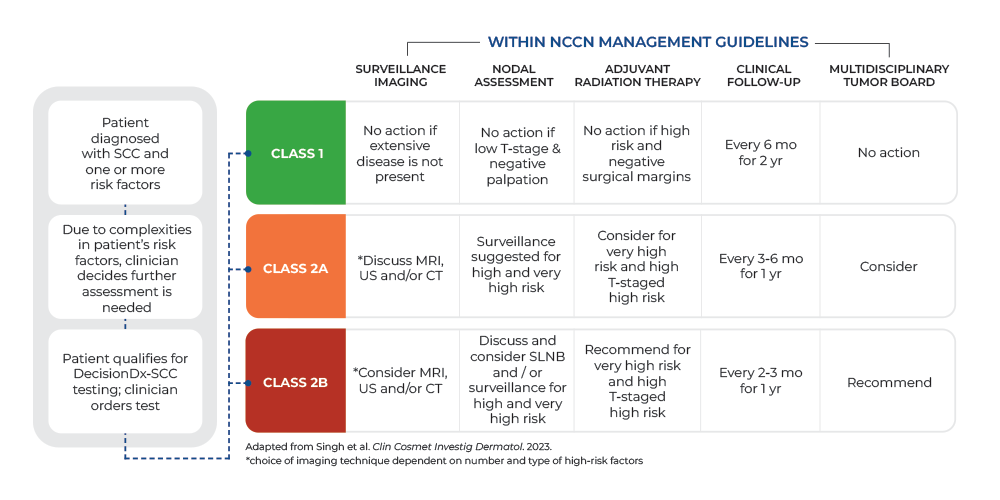
DecisionDx-SCC reporting
The DecisionDx-SCC report provides a class score based on a patient's biological risk:
Class 1: Low biological risk
Class 2A: Higher biological risk
Class 2B: Highest biological risk
Provides precise, personalized risk of metastasis to inform patient management
Get started with DecisionDx-SCC









